Medical Devices
SLM GmbH is an independend third person as defined by EG-Guideline 2017/745 about medical devices and 98/75/EG.
- Exchange-services for informations about our test results of medical devices.
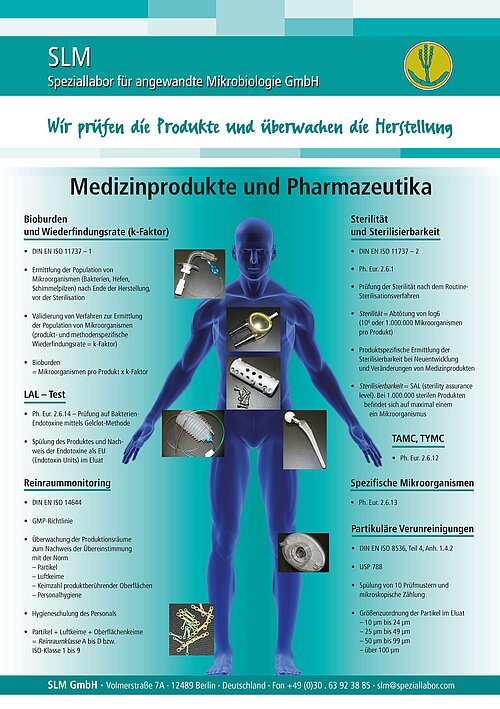
Example:Where are critical areas in the sterilization, cleaning and disinfecting process as well as when the product will be used later on and how could they be changed? - Testing sterility
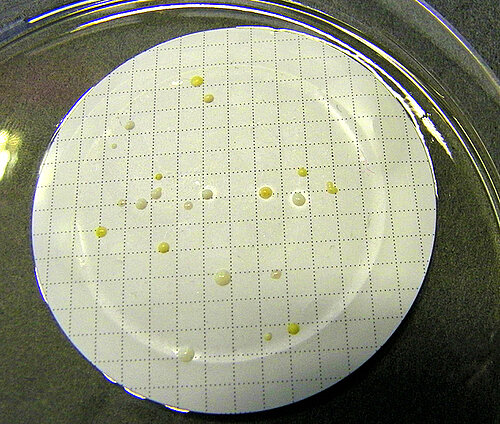
in accordance with DIN EN ISO 11737-2 and Ph.Eur. 2.6.1 - Determining the bioburden before sterilization
in accordance with DIN EN ISO 11737-1 - Determining the product- and method-specific recovery rate (k-factor)
in accordance with DIN EN ISO 11737-1 - Determining bacterial endotoxins with LAL test
in accordance with Ph.Eur. 2.6.14 methode A (gelclot) - Testing for particulate contamination
in accordance with DIN 8536, part 4, app.1.4.2 - Testing packaging material’s ability to maintain a sterile barrier
in accordance with DIN 58953-6 - AMES test
in accordance with OECD guideline for the mutagenic potential of materials (part of DIN EN ISO 10993-3)
Further Informations
PowerPoint-Presentations
- Bioburden: Nachweis und Wiederfindung (k-Faktor) > open (german)
- BEP: Prüfung auf Bakterien-Endotoxine nach Ph.Eur.2.6.14 (z.B. LAL-Test) > open (german)
- Validierung der Sterilisation von Medizinprodukten (Dr. Birgit Fiedler) > open (german)
- Reinigung – Desinfektion – Sterilisation bei der Wiederaufbereitung > open (german)
Frequently Asked Questions
- steril - Sterilisation - Prüfung der/auf Sterilität (PDF - german)
- Bioburden (PDF - german)
- k-Faktor - Wiederfindungsrate - Verfahrenseignung der Bioburden- Bestimmung (PDF - german)
- Bakterielle Endotoxine: Definition; Nachweis (BEP) z.B. mittels LAL-Test(PDF - german)
- Biokompatibilität (PDF - german)